Cleanroom & Critical Environments

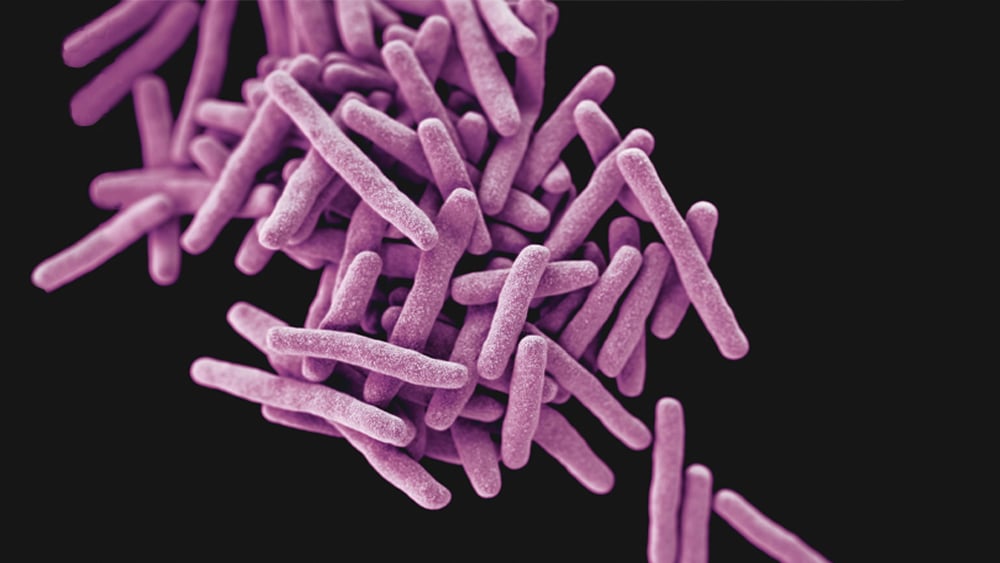
This article is also available in Spanish. Negative Pressure Rooms: A Key Component of Infection Control in Healthcare Negative pressure rooms have received increased attention in

This article is also available in Spanish. After a two-decades-long collaboration between international public and private sectors, the James Webb Space Telescope launched from Europe’s Spaceport

What equipment in your manufacturing environment is maintenance free? The answer is simple: none. As with all other equipment, ionizers used for static charge and
Precision is of utmost importance in the complex field of semiconductor equipment manufacturing and achieving perfection starts by establishing a controlled environment. At PAC, we

Cleanroom Wall System Installation Cleanroom Ceiling System Installation Completed Cleanroom & HVAC Installation Need Help with a Production Ready Cleanroom? PAC cleanroom specialists help you

This article is also available in Spanish. “Do more with less.” It’s the new mantra in today’s economy. Companies across many industries face the challenge
Aerosols vs Droplets – does COVID-19 spread by aerosols or droplets? Does evidence support airborne coronavirus? 15+ studies on COVID particle size, droplets, ventilation, and face masks.

Question: “What Class clean room is required to manufacture FDA approved N95 masks, isolation gowns, surgical masks, etc.?”.

This article defines, contracts, and compares FDA and EPA requirements for the efficacy of various chemical germicides, sterilants, and disinfectant chemicals.

What is GMP? What is CGMP? What are the differences between GMP and CGMP cleanrooms?

What are the options for temporary negative pressure triage and emergency room structures?

What is the proper technique for wipedown and sanitation of surfaces? Why is a microfiber wipe most effective for viruses and infection control?

What are the proper steps for wiping surfaces, biological isolators, and laminar flow cabinets, and excess hazardous drugs?

Cleanroom wipes and gloves undergo LAL and Agar Overlay Analysis when testing for pyrogen and endotoxin levels. This post details the methodology, historical relevance, and explores cleanroom solutions for pyrogen-free and low-endotoxin gloves and garments.

The majority of wiper contamination tests probe for particles, fibers, ions, nonvolatile residue, and bio-contaminants. Cleanroom wipe test methods detail and measure the extraction and enumeration particles, fibers, and other contamination released from wipers (or other materials), usually in a wetted state, under conditions of moderate mechanical stress.

The ideal cleanroom wipe must meet industrial and regulatory standards concerning chemical, physical, and biological compatibility. Wipe fabrics undergo a strict examination to simulate manufacturing outcomes in the real world.
Stretch and strength, chemical compatibility, task efficiency, sorbency, shed, retention, and biological harmony have unique consequences in both high-tech cleanrooms and general manufacturing floors.

ISO cleanroom wipe classification is important, but is it enough? A clean room wipe selection criteria should meet baseline particulate shed thresholds for the end-user environment. Like any cleanroom rated product, cleanroom wipe manufacturers qualify ISO wipers between ISO Class 1 – ISO Class 9 as specified in step with cleanroom classifications of the International Standards Organization (ISO). The majority of cleanroom classifications fall between ISO 5 – ISO 8.

Facilities often choose PAC for cleanroom construction services because our vendor and manufacturing networks are not limited to any single brand-specific solution. PAC cleanroom expertise in your back pockets helps you overcome preliminary engineering challenges and consolidate your project management to a single point of contact.

What are the steps for medical device cleanroom validation? Cleanroom validation ensures cleanroom construction meets the performance standards set forth during cleanroom planning, cleanroom installation, and cleanroom calibration.

Cleanroom tacky rollers and sticky foam rollers remove particulate from flat and slightly textured surfaces within a cleanroom. Extendable mop handles with adhesive rollers on the ends allow easy access to cleanroom, floors, ceilings, and walls.
