Table of Contents
Wipe Tests for Molecular Contamination, Particles, and Fibers
Cleanroom particulate tests and fiber enumeration measure airborne particles, fibers, and other releases from low lint wipes, textiles, or other cleanroom materials.
Particulate test methods describe the extraction and enumeration of particles and fibers released from wipers (or other materials) in a wetted state, under conditions of moderate mechanical stress.
Cleanroom Wipe Evaluation Quick Links
Liquid Particle Counting (LPC) Wipe Particulate Testing
Test Description: Particle Release from Wipers and Other Materials Under Conditions of Moderate Mechanical Stress
Preparing a sample for particle counting instruments or microscopic enumerations starts in an ISO 5 cleanroom. A controlled environment prevents extraneous contamination from producing false positives. The environment is similar to one required for sterile procedures. A laminar flow hood directs HEPA filtered air outward over the work surface and sweeps away trace contaminants from the working area.
When the LPC is set as outlined above, the unit will withdraw a 10 mL aliquot of fluid from the beaker (using the liquid sampling system that is part of the LPC) and determine the concentration of particles. The unit will repeat this process three more times. The software will discard the first reading and average the second, third and fourth results and report accumulative and differential particles per mL for all the established threshold (channel) ranges.
Texwipe TM22 6.2
Liquid particle counters (LPC) detect light scattered by small particles. Light is then turned into an electrical signal. Particles absorb light, which allows the machine to detect particles by inferencing the returned laser wavelength and light geometry.
Liquid Particle Counter Limitations
Historically, the limitations of particle testing equipment restricted liquid particle counts to 10,000 particles per mL in total. Samples containing larger particle counts required appropriate dilutions.
How Does Microscope Enumeration Count Particulates
A scanning electron microscope allows sample enumeration after agitating and stirring a wipe sample with the biaxial shake methods described IEST-RP-CC004. The sample is filtered and dried before evaluating the fiber counts under an optical microscope.
The use of SEM metrology for particle counting provides a method that is sufficiently sensitive to discern with great accuracy the number of particles released during sample preparation.3–5 However, the issue is how to prepare test samples in a manner that closely approximates the actual conditions of use invited further research.
Evaluating Sample Preparation Techniques for Cleanroom Wiper Testing 1997
Test Method 1.2) Fiber quantification is carried out in two ranges: 20 – 100 µm and > 100 µm. After extraction, fibers are captured on a gridded filter paper, let dry and then counted under an Optical microscope. Texwipe Test Method (TN22)
The challenge of this test is maintaining an ultra-pure extraction system.
Test Method 6.1): If the particles are not uniformly distributed over the filtered area of the filter, a new sample must be prepared. Uniformity of particle distribution over the filter is a must for accurate quantification. Texwipe Test Method (TN22)
Microscopy-based Measurement Technique for Liquid Particle and Fiber Counts
Test Description: Comprehensive Particle and Fiber Testing for Cleanroom Wipers November/December 1998
Electronic microscopes allow the enumeration of particles otherwise undetectable with an LPC for evaluating liquid particle counts, or APC used for airborne particle counts.
Novel wiper contamination tests from PAC vendors such as Texwipe showcase improved methods for enumerating particle and fiber counts within a single sample preparation.
The procedure uses a single sample preparation for the enumeration of all sizes, thereby eliminating the need for separate sample preparations for particles and fibers. The preparation involves immersing and agitating a wiper in a low-surface-tension cleaning liquid and filtering the particle-laden liquid through a microporous membrane filter.
The filter is then mounted on a scanning electron microscope (SEM) stub and first examined for the uniformity of distribution using the optical microscope. Once uniformity is determined, large fibers are also counted during this step. The sample stub is then transferred to the SEM, and particles of different size categories are counted at different magnifications. The accuracy and precision of the resultant counts are measured statistically.
The final results are reported with two different fiber sizes and totals.
LPC Results: >0.5 um
Fibers: >100 um
Related Documentation:
E2090-12, “Standard Test Method for Size-Differentiated Counting of Particles and Fibers Released from Cleanroom Wipers Using Optical and Scanning Electron Microscopy,” ASTM
Airborne Particulate Testing for Clean Room Garments and Wipes
Test description: IEST Recommended Practice RP-CC003.3: Garment System Considerations for Cleanrooms and Other Controlled Environments
The rolling barrel test is also known as the “Helmke Drum Test”. The method quantifies particle shed from a garment or wiper through a mechanical agitation under dry conditions. The fabric or material is placed in a stainless steel drum and rotated at 10 RPM. The drum is connected to a sampling tube and a laser particle counter.
Multichannel airborne particle counters allow particle detection and predetermined sizes, usually between 0.3, 0.5, 1.0 and 5.0 µm. Particle counts are taken at one-minute intervals for a period of 10 minutes.
IEST Clean Room Wipe Test Methods & Standards
Test Description: IEST-RP-CC004: Evaluating Wiping Materials Used In Cleanrooms And Other Controlled Environments
IEST-RP-CC004.4, describes methods for evaluating or testing wipers used in clean rooms and other controlled environments for characteristics related to both cleanliness and function.
Particle Enumeration
IEST-RP-CC004.3 describes two methods for removing particles from a wiper for enumeration, either laser-based liquid particle counter (LPC) or optical microscopy.
Enumeration is the act of establishing the number of ‘something’ in a swatch of fabric or use area. The testing, instruments, and enumeration methods are unique for small and large particle sizes, as well as target contamination of concern.
For cleanroom fabric particle tests, it’s the number of residual particles left over after a wipe is saturated in a solution, agitated, and removed. In other cases, testing requires larger, stranded fiber particle fibers.
IEST-RP-CC004.3 Minimal Stress Test
IES RP-004.2 – The minimal stress test immerses a wiper in a cleaner photographic tray filled with 500 ml of DI water. The wiper is gently agitated to ensure complete wetting. This test is a basis of comparison for other techniques with more rigorous test parameters.
A surfactant/DI-water mixture is often substituted with an isopropyl alcohol (IPA)/DI-water mixture to simulate an environment similar to end-use conditions.
IEST-RP-CC004.3: Biaxial Shake Test | Orbital Shake Test
Minimal Stress Test in a Low-Surface-Tension Environment
A biaxial shake test allows the detection of particles and fibers collected from a garment or wipe samples. It’s a well-explored test by leading cleanroom contamination organizations such as Texwipe, Kimberly-Clark, FG, and many more.
“The biaxial shake test described in IES RP-004.2 succeeds in imparting mechanical energy into the test environment as well as making the released particles available for counting in a liquid suspension.” (Texwipe 1997)
A laboratory shaker simulates repetitive motion and movement during clean room wipe use. A biaxial shaker is a lab instrument that mechanically stirs and mixes samples side-to-side- or up and down. Naturally, orbital shakers use programmable circular motions and allow aggressive motions without splash over. Orbital shakers contain turbulent liquids unlike biaxial shaking in a flat tray.
The test wipe is freely suspended in a tray of DI water, surfactant, or isopropyl alcohol solution. The ideal lab shaker for the test must consider what solution offers the most measurable fiber and test results in final evaluations.
When the final liquid sample is tested for particles by a Liquid Particle Counter (LPC), surfactants may generate bubbles and interfere with particle counting.
“When the motion is generated by a biaxial shaker, the wiper is extracted using water only. … When the motion is generated by an orbital shaker, a surfactant can be used since the extraction solution is filtered before analysis by optical particle counting and/or scanning microscopy.”
Differences Between Orbital Shake and Biaxial Shake for Liquid Particle Counting
Ideally, test results from orbital shaking methods are evaluated with a scanning electron microscope (SEM). Batch testing for biaxial shake tests will enlist a liquid particle counting method (LPC). This is often confusing when comparing cleanroom wipe cleanliness, as manufacturers may use different particle enumeration methods and non-translatable test mediums.
“The ability of the solutions to penetrate the interfaces between particles and surfaces affects both the surface to be cleaned and the wiper that is doing the cleaning. That is, if there are particles adhering to the wiper, they will be much more readily removed in a low-surface-tension environment than in pure DI water. “
Source: Texwipe – Evaluating-Prep-Techniques-Cleanroom-Wiper-Testing.pdf
Tests for Molecular Contamination and Ionic Content
High-performance Liquid Chromatography-Tandem Mass Spectrometry – HPLC-MS
High-performance liquid chromatography allows rapid testing for antineoplastic drugs. For example, the simultaneous detection of 5-fluorouracil, oxaliplatin, methotrexate, vindesine, ifosfamide, cyclophosphamide, vincristine, vinblastine, docetaxel, and paclitaxel.
Ion chromatography (IC)
The advent of high-sensitivity analytical instrumentation such as ion chromatography (IC), with its multi-ion separation and quantification capability, permits rapid and straightforward ion analyses at sub-ppm levels on a single extract
(Webb, 2009)
IC is a low cost, highly sensitive test which makes it common for ionic content testing.
Ion chromatography is a water chemistry analysis that measures concentration levels of anions and cations in parts-per-billion. IC wipe analysis narrows down a suite of target ions for testing. (F- , Cl- , NO2 – , Br- , SO4 2-, PO4 3-, Li+ , Na+ , NH4 + , K+ , Mg2+, Ca2+).
Anions and cations are then sampled at both normal and high temperatures. Its multi-ion separation and quantification capability permit rapid and straight forward ion analyses at sub-ppm levels on a single extract (Webb, 2009).
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
In this cleanroom wipe comparison from the Taiwan Association for Aerosol Research, cleanroom wipes are sampled for particles, ions, nonvolatile residue, and heavy metal concentration analytics via ICP-MS.
ICP-MS
Inductively coupled plasma mass spectrometry (ICP-MS) counts the number of ions at a fixed m/z. Heavy metal concentration is displayed as µg/g (micrograms per gram).
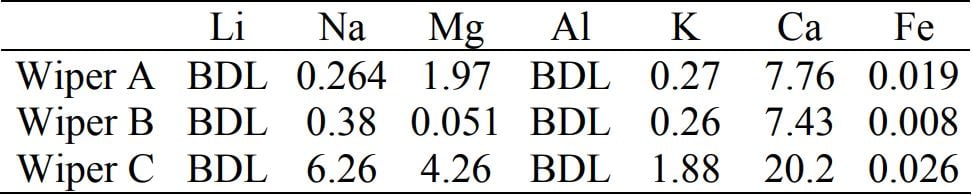
“Wiper B is an ISO 4 (Fed Class 10) cleanroom wiper, while wiper C is an ISO 5 cleanroom wiper (Fed. Class 100). The ISO 5 wiper contains over 10x more NVR than the ISO 4 wipe. BDL indicates “below detectable levels”.
What’s the Best Cleanroom Wipe for Each ISO Class or Industry?
Advanced cleanroom materials, environmental controls, and new generation cleanroom manufacturing techniques offer a scientific approach toward safer medical devices, pure medicines, and reliable component production.
In many cases, we field inquiries from customers who seek validated cleanroom supplies with reliable delivery. The questions are usually specific:
What is the best electronics wipe for an ISO 5 nano fab or wafer assembly environment?
What is the best wipe for a non-sterile medical device in an ISO 7 cleanroom?
What is the best absorbent wipe for chemical spills and solvent recovery?
The ideal clean room wiper is unique to device sensitivity, production components, and cleanroom application. Choosing the right cleanroom wipe for the right task often requires the assistance of a controlled environment specialist.
Wipes
Find dry, pre-saturated, and sterile wipes to meet strict demands of controlled cleanroom areas.
Mops
Cleanroom features include lint free fibers, multi-bucket antimicrobial features and no drip systems.
Disinfectants
EPA Registered disinfectants, effective against most pathogens. Fragrance & dye free.
Production Automation Corporation (PAC) is a factory-direct cleanroom supplier for environments, supplies, apparel, and furniture.
Every PAC product is backed by dedicated customer service, sales support, and in-house specialists.
Need help with a technical manufacturing solution? We’re the specialists in your back pocket.
Shop the PAC website or our CleanRoomProducts.com specialty division for factory-backed products curated by factory-trained specialists.
- We diligently choose suppliers and sell only direct authorized products
- We 100% stand behind all of our products
- Enjoy great pricing and great customer service
- Select from thousands of items on our site
- Customize your selection with accessories
- Get the best possible pricing available from any cleanroom specialty supplier
- Our product specialists have factory training on the items we sell

What’s the Correct Way to Wipedown a Surface? What’s the Advantage of Microfiber Wipes?
What is the proper technique for wipedown and sanitation of surfaces? Why is a microfiber wipe most effective for viruses and infection control?

How Do I Properly Wipe Down Surfaces in Cleanrooms and Labs?
What are the proper steps for wiping surfaces, biological isolators, and laminar flow cabinets, and excess hazardous drugs?

Particulate and Molecular Contamination Tests for Cleanroom Wipes and Fibers
The majority of wiper contamination tests probe for particles, fibers, ions, nonvolatile residue, and bio-contaminants. Cleanroom wipe test methods detail and measure the extraction and enumeration particles, fibers, and other contamination released from wipers (or other materials), usually in a wetted state, under conditions of moderate mechanical stress.

Cleanroom Wipe Contamination Profiles for Medical Device Wipedown & Electronics
The ideal cleanroom wipe must meet industrial and regulatory standards concerning chemical, physical, and biological compatibility. Wipe fabrics undergo a strict examination to simulate manufacturing outcomes in the real world.
Stretch and strength, chemical compatibility, task efficiency, sorbency, shed, retention, and biological harmony have unique consequences in both high-tech cleanrooms and general manufacturing floors.

Polycellulose & Polyester Cleanroom Wipe Supplies
Here’s the guide to buying cleanroom wipes that stay on-budget. Differing package sizes, bulk counts, edge types, material options, cloth size, weights, ISO class, substrates, ionic levels…comparing cleanroom wipes is mind numbing. 50% of leading cleanroom wipe advertisers don’t even offer prices online!

ISO 7 Cleanroom Panel Cleaning & Maintenance
Warm water and a mild dish soap is the best softwall curtain cleaning solution. What about acrylic, melamine, and aluminum? Generally, any biodegradable solution is acceptable. A 50/50 solution of Isopropyl Alcohol & DI water is also effective. Wash curtains front side and back, rinse with clean water, then wipe dry with a clean lint-free soft cloth.
Related Posts
-
New: Low Particulate Cleanroom Tape
Premium cleanroom tape and labels are feature non-particulating materials with plastic cores suitable for use in ISO 3 (Class 1) cleanrooms. Each roll and label is cleanroom bagged and packaged in a Class 100 (ISO Class…
-
CleanPro® Softwall Cleanroom Enclosure
This customer needed to enclose a piece of machinery, and CleanPro® was able to provide a solution.
-
CleanPro® Turnkey Cleanroom Install
CleanPro’s turn-key cleanroom solution provided a one-stop, one-contact result for the initial delivery and on-site installation of walls, ceiling grids, electrical systems, flooring, filters, HVAC, and more.
-
Cleanroom Mop Procedure - How to Mop a Cleanroom with a Multi-Bucket System
Learn how to properly mop a cleanroom for ISO class or cGMP cleanliness levels. Compare designs and materials of multi-bucket cleanroom mop systems.
-
Polycellulose & Polyester Cleanroom Wipe Supplies
Here's the guide to buying cleanroom wipes that stay on-budget. Differing package sizes, bulk counts, edge types, material options, cloth size, weights, ISO class, substrates, ionic levels…comparing cleanroom wipes is mind numbing. 50% of leading…
-
CleanPro® Softwall Cleanroom Curtains Installation
Softwall cleanroom curtains, sometimes referred simply as “plastic strips” yield ISO Class 10,000 level particle control with minimal construction. This customer required a custom softwall installation that integrated into their existing building structure CleanPro to…















