Table of Contents
Cleanroom wipes and gloves undergo LAL and Agar Overlay Analysis when testing for pyrogen and endotoxin levels. These tests ensure that cleanroom materials such as wipes or PPE do not introduce bacteria or pathogens into a cleanroom manufacturing process. This post details the methodology, historical relevance, and modern cleanroom solutions for pyrogen-free and low-endotoxin gloves and wipes.
Biological Contamination – Pharmaceuticals – Sterile Compounding – Lifesciences
Medical device and pharmaceutical cleanroom environments must address environmental exposure to bacteria, fungi, or viruses. Each common tool, material, or surface is capable of retaining microbiological artifacts, some inert, others parthenogenic.
Several investigators have recognized heavy microbial contamination of wet mops and cleaning cloths and the potential for spread of such contamination. They have shown that wiping hard surfaces with contaminated cloths can contaminate hands, equipment, and other surfaces.
Guideline for Disinfection and Sterilization in Healthcare Facilities (2008)
Read: Sterile Cleanroom Wipes | Endotoxin & Pyrogen Thresholds
Medical Devices, Pharmaceuticals, and Healthcare Wipe Contamination
In medical device and pharmaceutical cleanroom environments, providers must address environmental exposure to bacteria, fungi, or viruses. Repeated sterile outcomes are critical for applications such as sterile compounding, but also for test and research environments in laboratories and test facilities.
Every surface and tool is subject to a pragmatic and well-documented cleaning regimen that requires aggressive sterilants and certifiable products such as EPA registered, one-step disinfectants.
Example Wipedown Method for a Biological Safety Cabinet
Decontaminating critical tools or surfaces requires a multistep process with hyper-clean wipes, disinfectant agents, and sterile rinse water.
Remove visible particles, debris, or residue with proper solution
Use sterile, low-lint wipers. Identify the proper cleaning solution for your application.
Clean equipment and all interior surfaces of the PEC
Use Sterile Water for Injection or Sterile Water for Irrigation) using sterile, low-lint wipers
Apply a cleaning agent, followed by a disinfecting agent
Apply an EPA-registered one-step disinfectant. Ensure the contact time specified by the manufacturer is achieved.
Apply sterile 70% IPA to equipment and all interior surfaces
Using a low-lint wiper, apply sterile 70% IPA to equipment and all interior surfaces in the PEC.
Types of Contamination:
Biological contamination is a high risk for patients, especially those undergoing surgery recovery, the young and old, or those undergoing immunotherapies for cancer and autoimmune disorders.
“It’s a frequent precursor to human illness or discomfort (pg. 322) including infection, intoxication, and immunologic responses.”
James M. Seltzer, MD San Diego, Californi
Biological contamination may include:
- Bacteria
- Pyrogens & Endotoxins
- Fungal spores | Mycotoxins | Aflatoxin | Cytotoxicity
- Viruses & Pathogens
- Biofilms and residues
- Vermin, Insects,
- Saliva, Hair, Dander
- Heavy Metals
Read: Wipe Profiles for Medical Device Wipedown & Electronics
- Physical contamination: Fibers, particles, process materials
- Chemical contamination: vapor, gasses, moisture, molecules
- Biological contamination: fungi, bacteria, viruses
- Radioactive contamination
Read: Appropriate Assurance of Sterility for Medical Devices and Pharmaceuticals
What are Pyrogens and Endotoxins?
Pyrogens
Pyrogens include endotoxins which are derived from Gram-negative bacteria. Non-endotoxin pyrogens result from chemicals or microorganism-derived substances (source).
Endotoxins
“Bacterial endotoxins, also known as lipopolysaccharides, are a fever-producing by-product of gram-negative bacteria commonly known as pyrogens”. (Source)
Endotoxins are toxins released after the destruction of a host cell. Some endotoxins are classified as pyrogens. Pyrogens are molecules that produce fever.
Berkshire GW120.ST.15
Each package is gamma irradiated sterile to a 10^6 Sterility Assurance Level.
Passes LAL test for endotoxins <20 EU/device abrasion resistance and chemical compatibility required for cleaning critical sterile processing environments.
Gamma Wipe®120 is a knife cut, high sorbency cleanroom laundered wiper recommended for ISO Class 4 and above environments composed of 100% continuous filament polyester knit fabric.
USP Cleanroom Wipe Pyrogen Tests
Cleanroom wipes and gloves undergo LAL and Agar Overlay Analysis when testing for pyrogen and endotoxin levels.
A History of LAL and Pyrogen Test Methods
In the ’70s and ’80s, conversation stirred at the FDA over the sensitivity, reproducibility, scope, and simplicity of pyrogen test methods and lack of bacterial endotoxin limits. The “rabbit test” had been considered an adequate test method since its popular inception in 1925.
Scientists considered whether animal experiments were producing endotoxin tolerance. Common Gram-negative endotoxins such as Legionnaires’, which did not appear very pyrogenic during traditional rabbit testing, were shown 1,000 times more active during LAL tests. Was the rabbit test still considered an accurate indicator of potent toxins?
Throughout the mid-80s, the FDA continued discussions for more reliable and better-understood pyrogen test methods and made subsequent changes to bacterial endotoxin testing and product monograph requirements. By 1984, five USP water products were classified under specific bacterial endotoxin limits under the more reliable LAL tests. Most manufacturers of sterile water, sterile devices, and sterile medicines aligned toward validating Bacterial Endotoxin Tests.
Cleanroom Pyrogens – LPS & Gram-Negative Bacteria
Lipopolysaccharides (LPS) pyrogens may result in fever, shock, or death when introduced to the bloodstream, brain, or spine during medical procedures.
Historically, LPS (Lipopolysaccharides) is the most common pyrogen which emanates from the cell walls of gram-negative bacteria. (Texwipe – 1996 – Reducing Pyrogens in Cleanroom Wiping Materials). The USP, standards and oversight board for pharmaceutical and life science manufacturing, recognizes a shortlist of applicable tests for pyrogen and endotoxin screening.
Pyrogen Testing
Pyrogen testing is a requirement for all parenteral (injected) products including injectable vaccines. Today’s testing programs are the result of FDA test programs that identified objectionable endotoxin levels during drug and device analysis.
Pyrogen control requires starting with an extremely clean environment, entry barriers, and maintaining aseptic principles throughout production. Autoclaving and pore filtration are not universally effective in removing pyrogens and considered less ideal sterilization options than gamma irradiation. Sterile wipe suppliers often use gamma irradiation for final cleanroom wipe sterilization during manufacturing.
Bacterial Endotoxin Tests
The bacterial endotoxins test (BET) is a test to detect or quantify endotoxins from Gramnegative bacteria using amoebocyte lysate from the horseshoe crab (Limulus polyphemus or Tachypleus tridentatus). There are three methods for this test: •
Method A. The gel-clot technique, which is based on gel formation; •
Method B. The turbidimetric technique, based on the development of turbidity after cleavage of an endogenous substrate; •
Method C. The chromogenic technique, based on the development of color after cleavage of a synthetic peptide-chromogen complex.
World Health Organization
LAL (Limulus Amebocyte Lysate) Test for Cleanroom Bacteria Sampling

Limulus amebocyte lysate (LAL) is an endotoxin and lipopolysaccharide (LPS) test for Gram-negative bacteria.
LAL is of the more interesting cleanroom test contamination test methods. Specifically, the test uses bioindicators produced within the blood of horseshoe crabs. Amoebocytes are a blood extract retrieved from a horseshoe crab which clots and coagulates when encountering bacterial endotoxins. The blood is harvested from the crabs which are unharmed during the process and returned to their natural habitats.
Agar Overlay Analysis Cleanroom Wipe Test
An Agar Overlay test evaluates the diffusion of leachable chemicals from a sample. First, a wipe sample is overlaid on an L929 cell monolayer. A microscopic evaluation assesses how exposure to the wipe affects the cells active function. A neutral die indicates whether viable cells remain, or indicates cell stress or lysed cells.
“Cell lysis or cellular disruption is a method in which the outer boundary or cell membrane is broken down or destroyed in order to release inter-cellular materials such as DNA, RNA, protein or organelles from a cell.”
Multidisciplinary Digital Publishing Institute (MDPI).
The methodology for Agar Overlay techniques are examined within the United States Pharmacopoeia (USP) and the International Organization for Standardization 10993 compliant Agar Diffusion Test.
Cleanroom Pyrogen Sources: Gloves, Wipes, and People
A cleanroom wipe or wiper is an essential process tool for nearly every controlled manufacturing operation. Novel wipe materials allow repeatable cleaning among abrasive or delicate surfaces without introducing chemicals, fibers, particulates, or viruses and bacteria onto products, surfaces, and even people.
Studies show that bare hands and non-endotoxin free gloves yield higher endotoxin loading than samples collected wearing endotoxin-free gloves.
“Samples collected using cloths with bare washed hands yielded higher endotoxin loading per mass of collected dust versus samples collected wearing endotoxin-free gloves. This demonstrated additional endotoxin loading from the subject’s hand.”
Occupational and Environmental Health, College of Public Health, The University of Iowa
One must consider the use of wipes low in endotoxins, but also low endotoxin gloves. Glove-induced endotoxins may indicate positive results when transferred to contact sources.
Low Pyrogen Gloves
- These gloves are made with natural rubber latex, a renewable resource; Low protein and endotoxin
- Feature beaded cuffs for easy donning; AQL 1.5, exceeds ASTM standards for critical defects (pinholes)
- Double bagged with case liner, recommended for ISO Class 4 or higher cleanrooms
- Technical Data
Related: Hyflex, Nitrile, ESD and Cleanroom
Related: Accelerator & Sulfur Free Nitrile Exam Gloves
Related: Proper Materials for ESD Cleanroom Gloves
Advanced cleanroom materials, environmental controls, and new generation cleanroom manufacturing techniques offer a scientific approach toward safer devices and few complications.
The allowable limits of endotoxins in human and animal parenteral drugs, biological products and medical devices are established by the FDA
Poor quality control over glove sourcing and human-induced endotoxins may lead to inappropriately attributed contamination sources. Studies show cloths used with bare hands yield higher endotoxin loads by of collected dust versus samples collected wearing endotoxin-free gloves. Samples demonstrated that both hands and gloves introduce endotoxins to manufacturing processes.
Compare a wide selection from top brands and get bulk discounts. Need helping determining the proper garment or equipment for your gowning room? PAC provides controlled environment specialists with dedicated supply chain support.
Gram-negative Bacteria
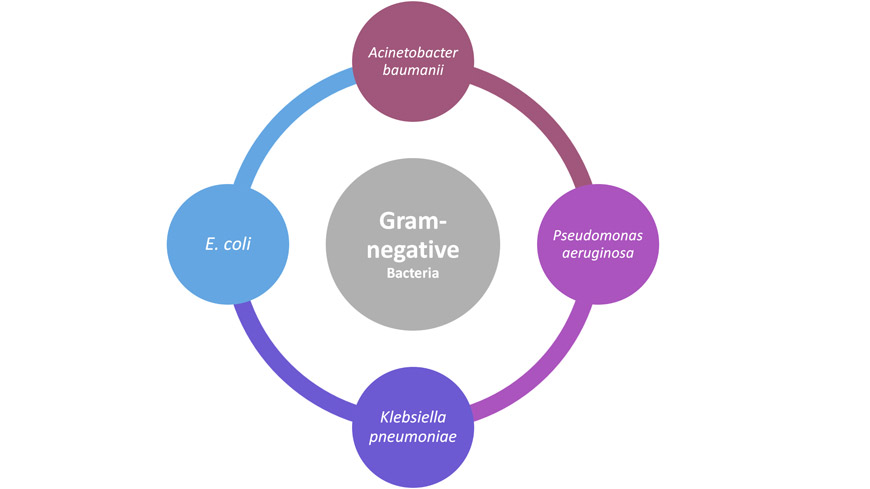
Problematically, Gram-negative bacteria are highly resistant to common cleanroom disinfection techniques such as 70% isopropyl alcohol. Heat, autoclave, and gamma sterilization are the most common sterilization techniques uses in cleanrooms.
Pseudomonas (e.g., P. aeruginosa) are the most frequent isolates from contaminated disinfectants—recovered from 80% of contaminated products. Their ability to remain viable or grow in use-dilutions of disinfectants is unparalleled.
Guideline for Disinfection and Sterilization in Healthcare Facilities (2008)
Wipe Sterilization Methods
- Wet steam autoclaving
- Electron Beam
- Steam Autoclaving
Best Method of Sterilization for Cleanroom Wipes
The allowable limits of endotoxins in human and animal parenteral drugs, biological products and medical devices are established by the FDA.
According to Texwipe, there are 5 reasons why gamma irradiation is chosen over other sterilization methods such as electron beam or autoclaving.
- Gamma irradiation has more penetrating power than electron beam
- Gamma irradiation is a relatively easy process to validate
- Gamma radiation leaves no residues from treatment.
- Gamma radiation is compatible with many wiper Texwipe wiper materials and products.
- Gamma irradiation has been shown to reduce endotoxin levels to a greater extent than electron beam irradiation.
Some nylons and polypropylenes are not compatible with the gamma irradiation process or high heat cycles.
Steam autoclaving requires a long high-intensity heat cycle required for a full microbial kill.
Most cleanrooms needing sterile outcomes resort to single-use consumables such as wipes, gloves, and garments. In sterile USP cleanrooms, disposable garments are regularly a requirement.
Sometimes, laundering and reusing cleanroom materials remains a more cost-effective strategy than disposables. However, one must consider the added cost, time, and labor of reusables besides comparing the cost vs single-use disposables.
What is Sterility Assurance Level?
“At present, a sterility assurance level (SAL) of 10–6 is generally accepted for pharmacopoeial sterilization procedures, i.e., a probability of not more than one viable microorganism in an amount of one million sterilized items of the final product.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2831250/
Affective SAL Levels for Medical Devices and Pharmaceuticals
SAL 10–6 for heat-resistant pharmaceutical preparations, suggested term: “Pharmaceutical sterilization”
SAL 10–4 for heat-resistant medical devices, suggested term: “High-level sterilization”
SAL 10–3 for heat-sensitive re-usable medical devices on the condition of prior validated cleaning efficacy >4 lg increments, suggested term: “Low-level sterilization
Proof of antimicrobial efficacy on the highest experimentally accessible level for all other products which, according to understanding to date, are to be applied sterile, suggested term: “Microbiologically safe for the designated use”
Institute for Hygiene and Environmental Medicine, University Greifswald, Germany
Most cleanroom facilities benefit from the assistance of a cleanroom contamination control specialist when sourcing and assessing contamination control strategies. Each surface of the cleanroom and its perspective decontamination task encounters various liquid volumes, different cleaning or disinfection needs, resistant microorganisms, and unique surfaces or abrasion factors.
Wipes
Find dry, pre-saturated, and sterile wipes to meet strict demands of controlled cleanroom areas.
Mops
Cleanroom features include lint free fibers, multi-bucket antimicrobial features and no drip systems.
Disinfectants
EPA Registered disinfectants, effective against most pathogens. Fragrance & dye free.
PAC (that’s us) is an end-to-end cleanroom supply distributor with locations and warehouses across the Americas. We’re your decontamination specialists. Compare top brands side by side at gotopac.com
Related Posts
-
Cleanroom Wipes for Pharmaceuticals: Pyrogen Testing Methods
Cleanroom wipes and gloves undergo LAL and Agar Overlay Analysis when testing for pyrogen and endotoxin levels. This post details the methodology, historical relevance, and explores cleanroom solutions for pyrogen-free and low-endotoxin gloves and garments.
-
Particulate and Molecular Contamination Tests for Cleanroom Wipes and Fibers
The majority of wiper contamination tests probe for particles, fibers, ions, nonvolatile residue, and bio-contaminants. Cleanroom wipe test methods detail and measure the extraction and enumeration particles, fibers, and other contamination released from wipers (or…
-
Cleanroom Wipe Testing Standards for ASTM, IEST & ISO Organizations
ISO cleanroom wipe classification is important, but is it enough? A clean room wipe selection criteria should meet baseline particulate shed thresholds for the end-user environment. Like any cleanroom rated product, cleanroom wipe manufacturers qualify…
-
New: Texwipe Cleanroom Products
Texwipe offers a variety of dry, pre-saturated, and sterile wipes to meet the strict demands of controlled environments. Texwipe swabs are manufactured to exacting and consistent tolerances with lot coded for traceability and quality control.
-
Cleanroom Mop Procedure - How to Mop a Cleanroom with a Multi-Bucket System
Learn how to properly mop a cleanroom for ISO class or cGMP cleanliness levels. Compare designs and materials of multi-bucket cleanroom mop systems.
-
Medical Device Cleanroom Build Part 7: Cleanroom Validation Steps | Processes
What are the steps for medical device cleanroom validation? Cleanroom validation ensures cleanroom construction meets the performance standards set forth during cleanroom planning, cleanroom installation, and cleanroom calibration.