Cleanroom USP Gowning Standards and Requirements
Cleanroom gowning requires different apparel based on the environment at hand. In every cleanroom, a minimum requirement includes shoe covers, hair nets (bouffants), and often a lab coat or frock.
In USP cleanrooms operator and patient safety are the most urgent risk factors. For example, sterile parenteral (for injection) drugs require a complete absence of microorganisms for safe administration to a patient (sterile outcomes). Therefore, sterile garments, laminar air flow hoods, and sterile PPE are prioritized.
USP Gowning Requirements
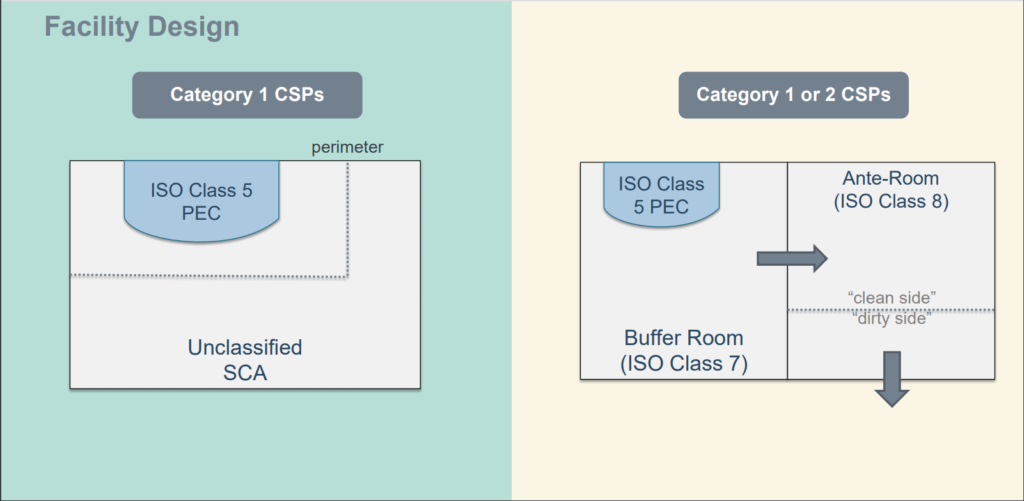
Proper Garments for Class 100 (ISO 5) – Class 10,000 Cleanrooms (ISO 7)
Cleanroom gowning protocol differs depending on cleanroom class and application. In ISO Class 7 or ISO Class 8 cleanrooms, frocks are often acceptable. A cleanroom classified as ISO Class 5 or ISO Class 6 (or cleaner) requires cleanroom coveralls, along with hoods, gloves, and booties (shoe covers). All gowns and garments should be rated by the cleanest process taking place, in USP cleanrooms, ISO 5 rated apparel is suggested.
For sterile processing, additional precaution is needed to assure that no sterile surfaces contact non-sterile surfaces during gowning, processing, or cleaning. Sterile garments and gloves are recommended during aseptic workflows. When hazardous compounds or chemicals are required, chemical resistant gloves, goggles, and aprons prevent operator exposure.
USP 797 Gowning and Garment Requirements
USP 797 gowning standards require extended consideration for proper gowning and personal protective equipment (PPE). The requirements may differ based on the State Board of Pharmacy. Disposable items like gloves, hoods, face masks, bunny suits, and shoe covers require safe and clean-forward disposal after every session. However, there are some cases in which certain gowns and garments may be reused.
USP 797 Sterile Gowning Revisions & Changes
The USP standard for gowning protocols is a target in motion. The below chart shows suggested revisions and previous standards. 2018 revisions for USP 797 are set for December, 1 2019. The final standard remains unknown.
In this case, the new suggestions reduce requirements for sterile sleeves and gowns. Most facilities currently using sterile apparel will continue to do so. All facilities should review its garment selection and revise with reference to the latest standards upon final release.
Expected USP Revision Timeline:
- Jun 2019: Revised USP 797 sent for publishing to USP-NF
- July 27, 2018: Revised comments republished for basic comment
- Dec 2019: Revised USP 797: Official release 6 months after publication in the USP-NF
| 2008 Revision | 2015 Revision | 2018 Suggested Revision |
| – Gown – Dedicated shoe covers – Head and facial hair covers – Face masks – Sterile gloves |
Determined based on: – Category – Type of PEC Includes: – Gown or coveralls – Disposable covers for shoes – Disposable covers for head – Disposable facial hair covers – Sterile gowns or sleeves – Sterile gloves |
– Gown – Disposable covers for shoes – Face mask – Sterile gloves If using RABS disposable gloves are allowed inside of gauntlet gloves |
USP 797 Sterile Compounding Requires:
- All gloves must be sterile and IPA compatible
- Sterile USP-grade Isopropyl alcohol or is required for intermittent disinfection of gloves.
- Allow 30 seconds of air drying for isopropyl alcohol kill time before resuming.
- High risk compounding requires the same garbing and gloves as required for ISO Class 5 processes
- Compounding personnel generally prefer gowns that snap or button in the front for easy use, but USP Chapter 797 does not specify a requirement.
USP 797 Does Not Require:
- Does not mandate change out of gloves which contact non-sterile surfaces but does require disinfection with sterile 70% isopropyl alcohol
- Proposed revisions indicate that sterile garments and sleeves will no longer be suggested in upcoming USP provisions starting December 2019. Those in question should review the latest revisions of USP documentation.
Workstation Requirements
| Laminar airflow system (LAFS) |
Laminar Airflow Workbench (LAFW) Integrated Vertical Laminar Flow Zone (IVLFZ) Class II Biological Safety Cabinet (BSC) |
| Restricted-access barrier system (RABS) | Compounding Aseptic Isolator (CAI) Compounding Aseptic Containment Isolator (CACI) |
General Gowning Order
USP 797 2019 Order of Donning and Gowning Sequence
The following excerpts outline the USP’s most recent responses to comments on the suggestions and best practices of gowning order and donning sequence:
Commentary Summary #74: Commenter requested details on how to don and doff
shoe covers in the cleanroom suite or SCA.
Response: Comment not incorporated. The order of garbing must be determined by the
facility and documented in the facility’s SOP. The order of garbing would depend on the
type of garbing used (e.g., sterile gowns) and the placement of the sink (e.g., if the sink
is located inside or outside of the ante-room). Further, the chapter states that garb must
be donned and doffed in an order that reduces the risk of contamination.
Commentary Summary #65: Several commenters requested a standard on the order
of garbing. Some commenters noted that the best order of garbing should be head
covers, face covers, shoe covers and then to step over the line from dirty to clean.
Response: Comment not incorporated: Garbing should be donned in an order that
proceeds from those activities considered the dirtiest to those considered the cleanest.
The order of garbing must be determined by the facility and documented in the facility’s
SOP. The order of garbing would depend on the type of garbing used (e.g., sterile
gowns) and the placement of the sink (see 4.4 Water Sources).
It is the responsibility of each facility to define and prioritize gowning order SOPs in a way that best reduces contamination risks.
USP 797 2019 – Garment Requirements – (Revision Bulletin) (Pg. 7)
Any person entering a compounding area must be properly garbed in accordance with the facility’s SOPs. Garb must be donned and doffed in an order that reduces the risk of contamination.
The order of garbing must be determined by the facility and documented in the facility’s SOP.
Sterile gloves must be donned in a classified room or SCA.
Skin must not be exposed inside the ISO Class 5 PEC (e.g., gloves must not be donned or doffed inside the ISO Class 5 PEC exposing bare hands).
Donning and doffing garb should not occur in the ante-room or the SCA at the same time. The minimum garbing requirements include:
• Low-lint garment with sleeves that fit snugly around the wrists and that is enclosed at the neck (e.g., gowns or coveralls)
• Low-lint, disposable covers for shoes
• Low-lint, disposable covers for head that cover the hair and ears, and if applicable, disposable cover for facial hair
• Face mask
• Sterile powder-free gloves
• If using a RABS, such as a CAI or CACI, disposable gloves (e.g., cotton, nonsterile, sterile) should be worn inside gloves attached to the RABS sleeves. Sterile gloves must be worn over gloves attached to the RABS sleeve.
Garb must be replaced immediately if it becomes visibly soiled or if its integrity is compromised.
Gowns and other garb must be stored in a manner that minimizes contamination (e.g., away from sinks to avoid splashing). When personnel exit the compounding area, garb except for gowns cannot be reused and must be discarded.
Gowns may be re-used within the same shift if the gown is maintained in a classified area or inside the perimeter of an SCA.USP 797 2008 (Revision Bulletin) (Pg. 29
Order of Compounding Garb and Cleansing: (Outdated)
Personnel shall don the following PPE in an order that proceeds from those activities considered the dirtiest to those considered the cleanest. Garbing activities considered the dirtiest include donning of dedicated shoes or shoe covers, head and facial hair covers (e.g., beard covers in addition to face masks), and face masks/eye shields. Eye shields are optional unless working with irritants such as germicidal disinfecting agents or when preparing hazardous drugs.
Personnel shall don the following PPE in an order that proceeds from those activities considered the dirtiest to those considered the cleanest. Garbing activities considered the dirtiest include donning of dedicated shoes or shoe covers, head and facial hair covers (e.g., beard covers in addition to face masks), and face masks/eye shields. Eye shields are optional unless working with irritants such as germicidal disinfecting agents or when preparing hazardous drugs.
After donning dedicated shoes or shoe covers, head and facial hair covers, and face masks, a hand cleansing procedure shall be performed by removing debris from underneath fingernails using a nail cleaner under running warm water followed by vigorous hand washing. Hands and forearms shall be washed to the elbows for at least 30 seconds with soap (either nonantimicrobial or antimicrobial) and water while in the ante-area. The use of antimicrobial scrub brushes is not recommended because they can cause skin irritation and skin damage. Hands and forearms to the elbows will be completely dried using either lint-free disposable towels or an electronic hand dryer.
After completion of hand washing, a nonshedding gown with sleeves that fit snugly around the wrists and enclosed at the neck is donned. Gowns designated for buffer area use shall be worn, and preferably they should be disposable. If reusable gowns are worn, they should be laundered appropriately for buffer area use.
- Dedicated cleanroom shoes or shoe covers
- Head and facial hair covers
- Face mask
- Fingernail cleansing
- Hand and forearm washing and drying
- Donn non-shedding gown
- Use water less alcohol-based surgical scrub
- Allow hands to fully dry
- Don sterile, powder-free gloves
- Disinfect gloves with sterile IPA, allow to fully dry
- Inspect for holes immediately and throughout
Personal Hygiene and Gowning Procedures
〈797〉 Pharmaceutical Compounding—Sterile Preparations Revision Bulletin 2008
<797> Pharmaceutical Compounding—Sterile Preparations 2019
Subject to Change
USP specifications are subject to change, as are organizational SOPs, and state by state requirements. For help regarding localized requirements, most locations benefit from contacting their State Board of Pharmacy. The content herein does not constitute a recommendation for your facility.
The proper cleanroom gowning room design, gowning supplies and equipment are essential for cleanroom contamination control. This informational guide should never take precedence over consulting with a cleanroom contamination specialist who is familiar with your specific equipment and classification needs. For help with cleanroom construction, gowning selection, or furniture purchases, our controlled environment specialists are available for a zero-cost consultation by phone or email.
Find Expertise
Find a Specialist
Free ConsultationCompare USP Garments
Shop Garments
Shop USP PPEThe content provided is for informational purposes only. It does not constitute a recommendation for your facility or organization. This post serves as a general guideline and summary. All information is subject to change and requires temporal review on a case-by-case basis according to state and federal law. The content herein, linked content or other web content provided by Production Automation Corporation (PAC) makes no claims as to the final interpretations or implementation of regulatory documents provided by the FDA, State Board of Pharmacy, or the United States Pharmacopoeia (USP). PAC provides on-staff environmental control specialists available every business day, toll-free (888) 903-0333 for consultation at no cost, no obligation.
Related Posts
-
USP Postpones USP 797 and USP 795 Deadline Until Further Notice (Nov 2019)
As of November 22nd, 2019, the USP will delay implementing previously suggested changes to USP 795 and 797 chapters until further notice.
-
USP Posts Revised PDFs for USP 795, 797, 800, 825 - Updated November 2019
Updated November 2019: Where can I find updated USP 800, 797, and other compounding documentation? Are there any recent changes to USP documents? When is the official deadline for USP 800 compliance?
-
USP <800> Compounding Cleanrooms
Updated: USP 800 is a cleanroom standard issued in March of 2014 by the United States Pharmacopeial Convention (USP). The deadline December 2019 for compliance may change.
-
Upgrading a USP 797 Cleanroom to USP 800?
New USP guidelines may present challenges for compounding facilities. Some facilities need infrastructural and mechanical modifications for compliance. System evaluation includes duct systems, HEPA fan filters, differential pressure standards, air monitoring, and external air exhaust…
-
USP 797 Guidelines: Sterile Compounding Cleanroom Design, Components, and Procedure
USP Chapter 797 cleanroom design requires that facilities pressurize non-hazardous compounding and storage areas. ISO 5, 7 and 8 environments support primary engineering controls, buffer rooms, and ante-rooms. Updated: 4/18/2019
-
How Does USP 800 Change Storage and Unpacking of Hazardous Drugs?
Facilities unpacking hazardous drugs within a negative pressure room do not require procedural changes to meet USP unpacking area requirements. Facilities unpacking HDs within positive pressure ante rooms or facility spaces will require reconsideration.
-
Updated: USP Sterile Compounding 2019
First published in 2004, USP Chapter has undergone proposed revisions as of July 2018. These revisions are now available for public comment.











1 thought on “USP 797 – Garbing and Donning”
Still not sure if putting cover shoes in garbing order is the best practice, I think it should come after head cover and mask:why? I think putting shoes cover first we may touch dirty shoes/ unless it is provided shoes scrubber/ and as bactirea and viruses can live and spread out of tissue, and we can accidantely spread them to hair and in face as we put the face mask, for that reason I think order should be. : Head cover, fase mask , beard cover, then shoes cover