Updated: 7/1/2020
United States Pharmacopoeia USP 797 took effect on January 1st, 2004 as a regulatory document which outlines procedures and environmental requirements for compounded sterile preparations (CSPs). Favorable outcomes in USP 797 cleanrooms also require proper laminar flow, workstation placement, operator technique, sanitation, and room air cleanliness.
USP 797 Revisions
2015 and 2018 revision proposals indicate a continually changing landscape for USP related mandates. One should always reference the most recent state regulations, internal SOP, and the most up to date USP chapter.
UPS 797 Non-Hazardous Cleanroom Design
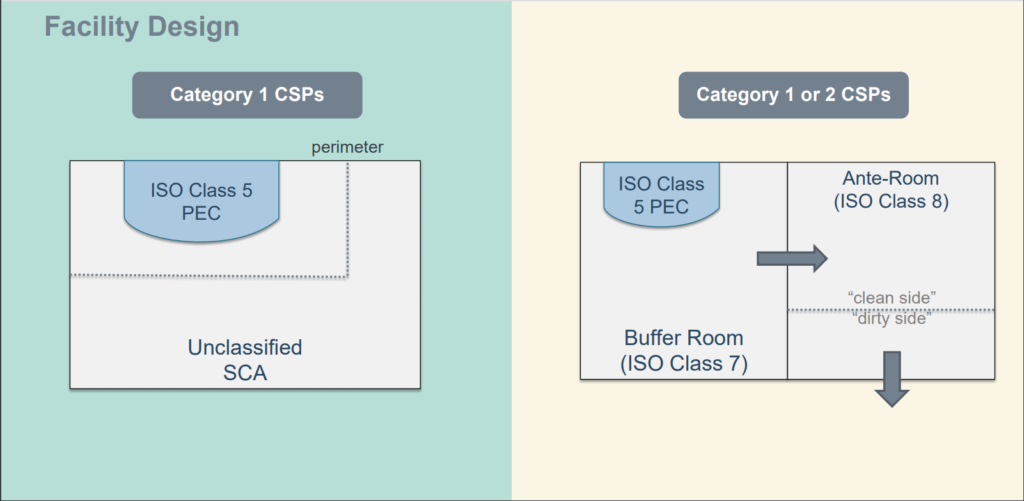
USP Chapter 797 cleanroom design requires that facilities pressurize non-hazardous compounding and storage areas. ISO 5, 7 and 8 environments support primary engineering controls, buffer rooms, and ante-rooms.
- ISO Class 5 Primary Engineering Control (external venting not required)
- ISO Class 7 Positive Pressure Buffer Area
- ISO Class 8 Positive Pressure Ante Room
Positive pressure ensures that in the case of a breached barrier, the space maintains sterility. Positive pressure rooms allow compounding of TPN (Total Parenteral Nutrition), antibiotic injections, eye-drops, infusion, syringes, salves, oils, and more. A direct compounding area with an ISO Class 5 laminar flow device requires no less than 30 ACH (air changes per hour).
USP 797 Hazardous Cleanroom Design (USP 800)
While USP 797 previously established requirements for hazardous drug compounding, USP Chapter 800 sets forth new requirements. USP 800 is the latest United States Pharmacopoeia (USP) revision which clarifies and expands upon hazardous drug compounding (both sterile and non-sterile). Enhanced engineering and procedural controls extend protection to operators and environments when packaging, handling, and compounding hazardous substances (including 2018 updates to the NIOSH list). USP 797 facilities compounding or handling hazardous drugs may require a number of new concessions including updated equipment, storage, engineering controls, and negative pressure environments.
What upgrades does a USP 797 facility require for USP 800 compliance?
- ISO Class 5 Primary Engineering Control (PEC)
- ISO Class 7 Negative Pressure Buffer Area (S-PEC)
- ISO Class 8 Positive Pressure Ante-Room
- 0.01″ of Water Column Relative to the Adjacent Area
OR
- An Equivalent CACI with Respect to Sterile vs Non-Sterile Parameters
Primary Engineering Control (PEC) Requirements
| Laminar airflow system (LAFS) | Laminar Airflow Workbench (LAFW) Integrated Vertical Laminar Flow Zone (IVLFZ) Class II Biological Safety Cabinet (BSC) |
| Restricted-access barrier system (RABS) | Compounding Aseptic Isolator (CAI) Compounding Aseptic Containment Isolator (CACI) |
What Type of Cleanroom Design do I Need?
Radiopharmaceuticals
USP 797 no longer covers measures for handling of radiopharmaceuticals. See USP 825.
USP 797 Hazardous Compounding Updates
While USP 797 previously established requirements for hazardous drug compounding, USP Chapter 800 sets forth new requirements. USP 800 is the latest United States Pharmacopeia (USP) revision which clarifies and expands upon hazardous drug compounding (both sterile and non-sterile). Enhanced engineering and procedural controls extend protection to operators and environments when packaging, handling, and compounding hazardous substances (including 2018 updates to the NIOSH list).
USP 797 facilities compounding or handling hazardous drugs may require a number of new concessions including updated equipment, storage, engineering controls, and negative pressure environments.
Negative pressure environments mitigate the risk for harm to both the environment and workers when compounding or packaging hazardous or toxic chemicals.
New USP guidelines may present challenges for compounding facilities. Some facilities need infrastructural and mechanical modifications for compliance. System evaluation includes duct systems, HEPA fan filters, differential pressure standards, air monitoring, and external air exhaust equipment. Learn more.
The USP compounding guideline, Chapter 800, clarifies and expands upon the hazardous drug guidelines found in USP 797. USP 800 expands controls for the protection of workers and environments against hazardous drug compounds. In contrast to USP 797, which only remedies sterile compounding activities, USP 800 takes a 360° approach for processing hazardous drugs, both sterile and nonsterile.
Previous to USP 800, USP 797 made some exemptions for small volumes of HDs (hazardous drugs) compounded in a positive pressure room when using a negative pressure laminar flow workstation or compounding aseptic containment isolator (CACI). These exceptions were generally removed by the USP 800 revisions for facilities storing, handling, and processing HDs.
USP 797 Cleanroom Requirements: Furniture, Ceilings, Walls Floors, and Process Support Equipment
All furniture and materials that enter the area must be non-permeable, non-shedding, cleanable, and resistant to disinfectants. Ceilings, walls, floors, shelving, fixtures, cabinets, pass-throughs, and counters must also support cleaning, be non-shedding, and remain free of cracks and crevices that hinder sanitation.
Cleanrooms have limited space. Factoring in the footprint, ergonomics and aseptic management of various equipment, tools, and furniture requires help from cleanroom design experts. The placement of a sink, bench, storage cabinet or doorway may hinder effectiveness or disrupt laminar flow. The final cleanroom design must consider all essential processes, procedures, and personnel.
USP 797 Compliance: Gowning, Garbing, and PPE
USP 797 standards require consideration for proper gowning and personal protective equipment (PPE). The requirements may differ based on the State Board of Pharmacy. Some items like gloves, hoods, face masks, bunny suits, and shoe covers require safe and clean-forward disposal after every session. Cleanroom dispensers and receptacle arrangements are essential for throughput and efficient workflow. Less movement means faster task completion and reduced particulate generation.
Automated gowning is popular for facilities with limited space, but also for high-volume facilities. Automatic shoe cover dispensers and removers ease congestions, cut bottlenecks and transfer fewer particulates during the gowning process.
Need Cleanroom Design Help at No Cost?
Upgrading or Building a New Cleanroom?
CleanPro®, a division of Production Automation, is a trusted ally of enterprises, research facilities, and government agencies. We leverage 100 years of cleanroom experience to draft, design, schedule, and deliver production ready cleanrooms.
The content provided is for informational purposes only. It does not constitute a recommendation for your facility or organization. This post serves as a general guideline and summary. All information is subject to change and requires temporal review on a case-by-case basis according to state and federal law. The content herein, linked content or other web content provided by Production Automation Corporation (PAC) makes no claims as to the final interpretations or implementation of regulatory documents provided by the FDA, State Board of Pharmacy, or the United States Pharmacopoeia (USP). PAC provides on-staff environmental control specialists available every business day, toll-free (888) 903-0333 for consultation at no cost, no obligation.
Related Posts
-
USP 797 - Garbing and Donning
View USP 797 gowning standards and core requirements. For sterile processing, additional precaution is needed to assure that no sterile surfaces contact non-sterile surfaces during gowning, processing, or cleaning. Sterile garments and gloves are recommended…
-
USP <800> Compounding Cleanrooms
Updated: USP 800 is a cleanroom standard issued in March of 2014 by the United States Pharmacopeial Convention (USP). The deadline December 2019 for compliance may change.
-
Updated: USP Sterile Compounding 2019
First published in 2004, USP Chapter has undergone proposed revisions as of July 2018. These revisions are now available for public comment.
-
Upgrading a USP 797 Cleanroom to USP 800?
New USP guidelines may present challenges for compounding facilities. Some facilities need infrastructural and mechanical modifications for compliance. System evaluation includes duct systems, HEPA fan filters, differential pressure standards, air monitoring, and external air exhaust…
-
Cleanroom and Sterile Compounding Glossary and Definitions
The definitive cleanroom and sterile compounding glossary. Learn terminology and definitions for USP 797 and USP 800 compounding.
-
CleanPro® Chemotherapy & CSP Cleanroom Installation
This modular, sterile compounding cleanroom is designed for USP 797 and USP 800 compliance, particularly for compounding chemotherapy drugs. Safe handling of sterile compounds requires special considerations: heat-welded floors, anterooms and buffer areas.