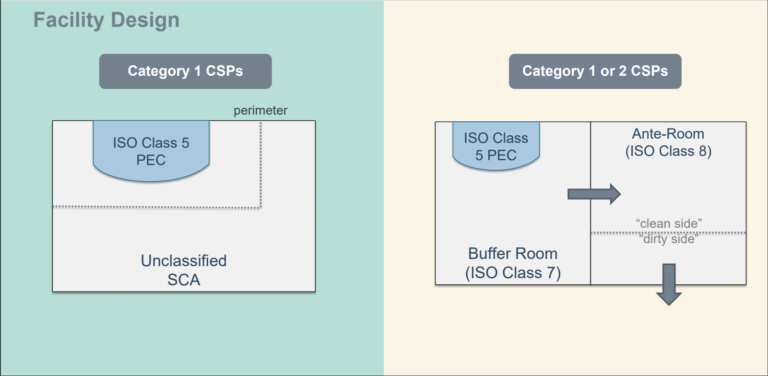
Cleanroom Wipes for Pharmaceuticals: Pyrogen Testing Methods
Cleanroom wipes and gloves undergo LAL and Agar Overlay Analysis when testing for pyrogen and endotoxin levels. This post details the methodology, historical relevance, and explores cleanroom solutions for pyrogen-free and low-endotoxin gloves and garments.