On June 1, 2019, the USP published revisions to USP 795 and USP 797 and established a final rollout date of December 2019.
As of November 22nd, 2019, the USP will delay implementing previously suggested changes to USP 795 and 797 chapters until further notice.
Official Statement on USP 795 | USP 797 Changes & Documents
“In light of these appeals, and in accordance with our Bylaws, USP is postponing the official date of <797> until further notice. In the interim, the current official version of <797> (last revised in 2008) including the section Radiopharmaceuticals as CSPs will remain official. The decisions on the appeals to <797> do not foreclose the possibility of future revisions to this chapter.”
USP.org
Currently Official USP 797 & USP 795 Standards As of November 2019
As of November 22nd, 2019, USP guidelines and standards remain in effect by guidelines of the previous version:
USP 797: “The currently official version of General Chapter <797> (last revised in 2008) remains official until further notice.”
USP 795: ” n the interim, the currently official version of <795> (last revised in 2014) will remain official.”
-USP.org
Changes and Revisions (22–Nov–2019)
The USP reports 8,000 asseverations from industry stakeholders during its public comment period, the USP responded to a number of suggestions.
The comments reflected diverse inputs from organizations, academics, regulators, practitioners, pharmacists, and representatives.
Comments considered as appeals to <795> and <797> revisions included:
- Beyond-Use Date (BUD) provisions in <795> and <797> `
- Removal of Alternative Technology provision from <797> `
- Applicability of <795> and <797> to veterinary practitioners
Reinstate the Alternative Technology Provision USP 797 2008
“The CMP EC recognized that <797> may not capture all modalities used in pharmacy compounding. However, the CMP EC also intends to publish Frequently-Asked-Question (FAQ) to clarify that the reinstatement of the Alternative Technology provision is not intended to permit BUD extension or to extend the time during which single-dose containers may be used.”
Notably, the USP maintains that it does not intend to allow provisions for BUD or single-use container times.
USP Honors Opportunity for Further Review of Appeals
“In accordance with the Rules and Procedures of the 2015–2020 Council of Experts, USP is postponing the official date of Pharmaceutical Compounding—Nonsterile Preparations <795>.”
“As part of the formal USP appeals process, stakeholders who submitted appeals to the compounding chapters had the opportunity to request a further review by an appointed Panel, and USP has received three (3) such requests on <797>.”
USP Maintains Veterinary References as a “Best Practice Standpoint”
“<795> and <797> do not state compendial requirements for animal drug compounding under federal law. Section 503A of the Federal Food, Drug and Cosmetic Act, which makes <795> and <797> applicable to pharmacy compounding, applies only to pharmaceuticals for human use.”
“<795> and <797> contain provisions that are intended to be relevant and useful for veterinary practitioners. For this reason, it is the CMP EC’s view that continued reference to veterinarians in both <795> and <797> may serve value, from a best practice standpoint. The requirements of these chapters are relevant to ensuring quality CSPs for both human and animal patients.”
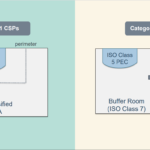
BUD – CSP Cleanroom Categories
Beyond-Use-Date does have implications on the requirements of a cleanroom suite. For example, Category 1 and Category 2 CSPs have unique cleanroom environments and BUD standards.
Category 1: CSPs are typically prepared in an unclassified Segregated Compounding Area (SCA) and have shorter BUDs.
An unclassified SCA is a controlled environment without a specified air cleanliness rating. An SCA provides most of the same pressure requirements but does not require a specific threshold of particles per square meter.
Category 2: CSPs are prepared in a cleanroom suite and have longer BUDs.
Implications for Small Pharmacies, Hospitals, Procedural Areas, or Clinics
PAC (that’s us) frequently fields inquiries from small pharmacies and clinics who are sometimes in a feverish hurry to understand how USP 797 or USP 800 revisions impact their facility. Further, they want to know what types of architectural or infrastructure upgrades they’ll require for continued USP cleanroom compliance.
- What are the Differences Between USP 795, 797, and 800?
- How Does USP 800 Change Storage and Unpacking of Hazardous Drugs?
- USP 800 Cleanroom Standards Overview
- Upgrading a USP 797 Cleanroom to USP 800?
- USP 800 Cleanroom Construction Options and Alternatives
Need Help with a USP Cleanroom Build or Upgrade?
Production Automation is a trusted ally of enterprises, research facilities, and government agencies. We leverage 100 years of cleanroom experience to draft, design, schedule, and deliver production ready cleanrooms.
Related Posts
-
USP Posts Revised PDFs for USP 795, 797, 800, 825 - Updated November 2019
Updated November 2019: Where can I find updated USP 800, 797, and other compounding documentation? Are there any recent changes to USP documents? When is the official deadline for USP 800 compliance?
-
USP Posts Revised PDFs for USP 795, 797, 800, 825 - Updated November 2019
Updated November 2019: Where can I find updated USP 800, 797, and other compounding documentation? Are there any recent changes to USP documents? When is the official deadline for USP 800 compliance?
-
USP <800> Compounding Cleanrooms
Updated: USP 800 is a cleanroom standard issued in March of 2014 by the United States Pharmacopeial Convention (USP). The deadline December 2019 for compliance may change.
-
Upgrading a USP 797 Cleanroom to USP 800?
New USP guidelines may present challenges for compounding facilities. Some facilities need infrastructural and mechanical modifications for compliance. System evaluation includes duct systems, HEPA fan filters, differential pressure standards, air monitoring, and external air exhaust…
-
How Does USP 800 Change Storage and Unpacking of Hazardous Drugs?
Facilities unpacking hazardous drugs within a negative pressure room do not require procedural changes to meet USP unpacking area requirements. Facilities unpacking HDs within positive pressure ante rooms or facility spaces will require reconsideration.
-
CleanPro® Chemotherapy & CSP Cleanroom Installation
This modular, sterile compounding cleanroom is designed for USP 797 and USP 800 compliance, particularly for compounding chemotherapy drugs. Safe handling of sterile compounds requires special considerations: heat-welded floors, anterooms and buffer areas.
-
Updated: USP Sterile Compounding 2019
First published in 2004, USP Chapter has undergone proposed revisions as of July 2018. These revisions are now available for public comment.
-
Guide to USP <1072> Disinfectants & Sporicides
USP outlines decontamination practices for critical environments.In this post, we'll outline the types of chemical disinfectants and sterilants used in cleanrooms and laboratories. This includes isopropyl alcohol, bleach, formaldehyde, hydrogen peroxide, and peracetic acid.
-
Upgrading a USP 797 Cleanroom to USP 800?
New USP guidelines may present challenges for compounding facilities. Some facilities need infrastructural and mechanical modifications for compliance. System evaluation includes duct systems, HEPA fan filters, differential pressure standards, air monitoring, and external air exhaust…
-
USP 797 - Garbing and Donning
View USP 797 gowning standards and core requirements. For sterile processing, additional precaution is needed to assure that no sterile surfaces contact non-sterile surfaces during gowning, processing, or cleaning. Sterile garments and gloves are recommended…
-
USP 797 Guidelines: Sterile Compounding Cleanroom Design, Components, and Procedure
USP Chapter 797 cleanroom design requires that facilities pressurize non-hazardous compounding and storage areas. ISO 5, 7 and 8 environments support primary engineering controls, buffer rooms, and ante-rooms. Updated: 4/18/2019
-
USP 800 Cleanroom Construction Hazardous Compounding: Options and Alternatives
There are a few options available to cleanroom facilities who require new upgrades for USP 800 compliance. The best option will depend on the variables of the facility and cleanroom in question. Option A: Convert…
-
USP 800 Cleanroom Design - Negative Pressure Changes and Requirements
What equipment and engineering controls do USP 800 cleanrooms require? What types of storage and monitoring systems will I need?
-
USP 800 Gowning - Chemotherapy Gloves, Gowns
What are the essential requirements for USP 800 gowning? What kind of gloves and gowns do USP 800 hazardous drugs require?
-
USP <800> Compounding Cleanrooms
Updated: USP 800 is a cleanroom standard issued in March of 2014 by the United States Pharmacopeial Convention (USP). The deadline December 2019 for compliance may change.