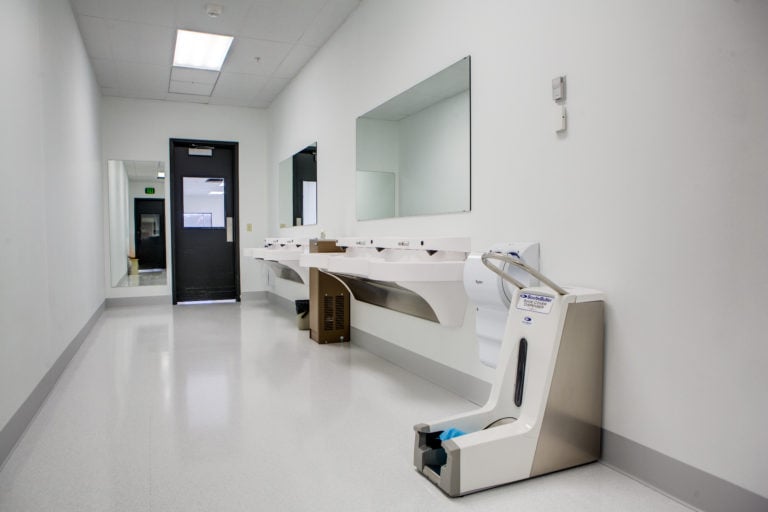
How CleanPro® Makes Common Items into Critically Compliant Cleanroom Equipment
Find hand dryers, laboratory receptacles and cleanroom mirrors. New from CleanPro with quick ship options.

Find hand dryers, laboratory receptacles and cleanroom mirrors. New from CleanPro with quick ship options.
ISO 13485 is designed for use throughout the life cycle of a medical device. It supports each stage of medical device development and operation from initial concept to production and disposal. The standard helps internal and external parties certify and validate processes which maintain the stability of quality management systems (QMS).

Lynx EVO is an ergonomic stereo microscope without eyepieces, powering your productivity through stunning 3D viewing.

What makes MAPA gloves popular in demanding conditions? We tested the full range of MAPA gloves for form, fit and function and highlight our favorites from each category.

Select your BenchPro workbench, and save 15% on any anti-fatigue mat or Lissner chair.
Perfex cleanroom mop and disinfection systems are designed for easy use and maintenance for critical operations. It’s the cleaning performance you’d expect beyond old-style buckets, wringers, string and sponge mops.

Find Creform alternatives at a much lower cost, sometimes, as low as half the price. Browse Proform pipe and joint configurations and get professional design expertise at no cost.

Are the terms sterile and aseptic used interchangeably? Is a cleanroom sterile? What is aseptic processing?

Cleanroom particle counters and anemometers offer portability, accuracy, and easy use. The self-contained devices allow local and off-site testing of air quality, air speed, HVAC efficacy.

Pre-engineered fabrication consolidates scheduling with minimal disruption and fewer variables. Difficult or expensive upgrades avoid overbearing noise, construction artifacts, and minimize if not eliminate the burden on facility staff for project management.

USP Chapter 797 cleanroom design requires that facilities pressurize non-hazardous compounding and storage areas. ISO 5, 7 and 8 environments support primary engineering controls, buffer rooms, and ante-rooms. Updated: 4/18/2019

New USP guidelines may present challenges for compounding facilities. Some facilities need infrastructural and mechanical modifications for compliance. System evaluation includes duct systems, HEPA fan filters, differential pressure standards, air monitoring, and external air exhaust equipment.
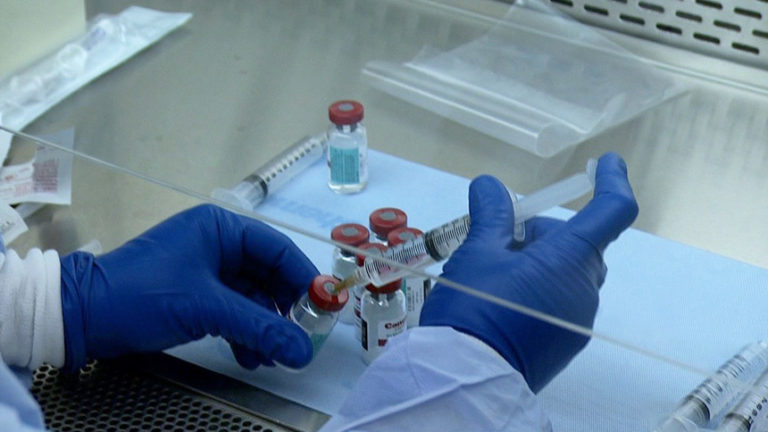
Facilities unpacking hazardous drugs within a negative pressure room do not require procedural changes to meet USP unpacking area requirements. Facilities unpacking HDs within positive pressure ante rooms or facility spaces will require reconsideration.
