The Role of Negative Pressure Rooms in Modern Healthcare
This article is also available in Spanish. Negative Pressure Rooms: A Key Component of Infection Control in Healthcare Negative pressure rooms have received increased attention in

This article is also available in Spanish. Negative Pressure Rooms: A Key Component of Infection Control in Healthcare Negative pressure rooms have received increased attention in

What is GMP? What is CGMP? What are the differences between GMP and CGMP cleanrooms?

What are the options for temporary negative pressure triage and emergency room structures?

ISO cleanroom wipe classification is important, but is it enough? A clean room wipe selection criteria should meet baseline particulate shed thresholds for the end-user environment. Like any cleanroom rated product, cleanroom wipe manufacturers qualify ISO wipers between ISO Class 1 – ISO Class 9 as specified in step with cleanroom classifications of the International Standards Organization (ISO). The majority of cleanroom classifications fall between ISO 5 – ISO 8.

What are the steps for medical device cleanroom validation? Cleanroom validation ensures cleanroom construction meets the performance standards set forth during cleanroom planning, cleanroom installation, and cleanroom calibration.

As of November 22nd, 2019, the USP will delay implementing previously suggested changes to USP 795 and 797 chapters until further notice.

What are the advantages of cleanrooms for e-liquids? What are the American E-liquid standards for the FDA, and ISO cleanrooms? Does the FDA require a cleanroom for e-liquids and e vape products?

What equipment and engineering controls do USP 800 cleanrooms require? What types of storage and monitoring systems will I need?

What is the proper way to calculate ISO 5 cleanroom air change rates? View ISO air velocity requirements and cleanroom benchmarks. (Class 100)

Updated November 2019: Where can I find updated USP 800, 797, and other compounding documentation? Are there any recent changes to USP documents? When is the official deadline for USP 800 compliance?
Large clinical-scale cleanroom operations for T cell therapies often use a class ISO 7 (Class 10,000) cleanroom room environment with an ISO 5 (Class 100) biological safety hood.
What Kind of Cleanroom Do I Need for a Class II Medical Device? What About Class III? This post classifies medical device cleanrooms for manufacturing and packaging.

First published in 2004, USP Chapter <797> has undergone proposed revisions as of July 2018. These revisions are now available for public comment.
ISO 13485 is designed for use throughout the life cycle of a medical device. It supports each stage of medical device development and operation from initial concept to production and disposal. The standard helps internal and external parties certify and validate processes which maintain the stability of quality management systems (QMS).

Updated: USP 800 is a cleanroom standard issued in March of 2014 by the United States Pharmacopeial Convention (USP). The deadline December 2019 for compliance may change.

USP Chapter 797 cleanroom design requires that facilities pressurize non-hazardous compounding and storage areas. ISO 5, 7 and 8 environments support primary engineering controls, buffer rooms, and ante-rooms. Updated: 4/18/2019
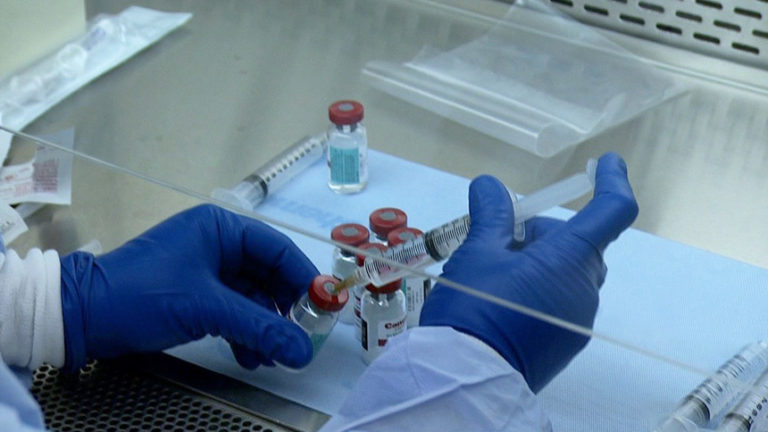
Facilities unpacking hazardous drugs within a negative pressure room do not require procedural changes to meet USP unpacking area requirements. Facilities unpacking HDs within positive pressure ante rooms or facility spaces will require reconsideration.

A comparison of cleanroom classifications and specifications.

Proper cleanroom cleaning procedure and maintenance protocol is an immediate, low-cost measure to enhance overall cleanliness, consistency, and contamination control within manufacturing and aseptic cleanrooms. This guide provides a framework for cleanroom management, protocol standards, specifications, and processes for general manufacturing and bio-medical applications.

Advantages for Modular Hardwall Design and Construction of ISO 14644-4 Cleanrooms.
