Table of Contents
Electronic Cigarettes, Vaporizers, and ENDS Devices
Cleanrooms provide a pharmaceutical-grade lab environment for E-liquid manufacturing [e-vape | e-juice | e-cigarette | e-salts | ENDS] and other tobacco-related vaporized compounds or devices.
Any substance intended for human consumption and sold on US markets requires manufacturing controls. Despite the mainstream popularity of electronic vaporizers and widespread use of vaporized E-liquids, manufacturing standards remain ambiguous.
Every production area is unique. Below we’ll contrast the benefits of each system.
E-Liquid Manufacturing Controls – Why Cleanrooms?
Cleanroom technology is adopted for a number of reasons. In non-regulated industries, cleanroom workflows and environmental controls subdue aerosol and surface contamination. Nearly every manufacturer or mixing/packaging retailer uses some type of cleanroom or clean area.
The area could consist of a temporary softwall curtain structure or a sprawling hardwall facility for mass assembly and packaging. In retail environments, a small containment area is often favored for custom e-juice mixes and preparing other batch ingredients for final packing. Some require large-scale environments for high-volume, and often automated filling, packaging, and bottling lines.
Cleanroom environments standardize quality control beyond the capabilities of non-controlled manufacturing environments. Controlled environments have many obvious perks: fewer batch-to-batch variables, cleaner end-products, and limited deviation of as-labeled ingredients. For example, a cleanroom monitoring system is capable of recording, logging, and sounding alarms when environmental conditions show unacceptable levels of airborne contamination, humidity, temperature, C02, or even toxic VOCs/aerosols. Manufacturers then correlate the time and severity of the event to ongoing procedures, such as times when operators entered the room, stages of equipment use, operator activity, state of HVAC conditions, and intervals between cleaning and decontamination sessions.
Before we cover the specific e-liquid cleanroom designs and options, let’s briefly cover the benefits.
Understand Production Variables
Controlled environments minimize environmental deviation that would otherwise skew data-driven conclusions between the perceived manufacturing environment and the actual environment. In some cases, lackluster humidity and temperature control may, prevent adhesives from curing, reduce the solubility of mixtures/solutions, increase machine maintenance costs, and affect material stability. All of these factors affect the final quality, defect rates, and shelf life of the final product. Room temperature and humidity also influence the productivity and comfort of operators and technicians.
Reduce Cleaning Burdens (Clean Faster in Less Time)
Cleanroom friendly chairs, tables, and work surfaces minimize cracks, crevices, and seams. Smooth, solvent compatible surfaces such as stainless steel are most common for easier cleaning with common disinfectants such as isopropyl alcohol, bleach, and hydrogen peroxide formulas.
Wipes
Find dry, pre-saturated, and sterile wipes to meet strict demands of controlled cleanroom areas.
Mops
Cleanroom features include lint free fibers, multi-bucket antimicrobial features and no drip systems.
Disinfectants
EPA Registered disinfectants, effective against most pathogens. Fragrance & dye free.
Prevent Residual and Human-Borne Contamination
Controlled environments standardize production quality and prevent manufacturing-induced contamination. This includes human-induced burdens such as skin cells, hair, bacteria, mucus, ect; but also, machine and environmental burdens such as dirt, debris, VOCs, manufacturing byproducts, residual contamination, biofilms, and more. Most sanitary areas restrict access to only operational staff.
The first step toward a clean environment is a sanitary one. One must address any sources of vermin, mites, pests, or insects. This also means the area is free of any carriers, such as pets, live plants, organic debris, and sitting moisture.
Meet Demand for Manufacturing Transparency
Customers show a growing demand for transparency, safer ingredients, and batch-to-batch consistency. Controlled manufacturing is not only a pillar of safe products but also ensures the best possible case for a positive user experience, i.e; more consistent flavor profiles, predictable vaporization temperature, better taste, longer shelf life, etc.
Differentiate Product Quality
A cleanroom production environment signals one of the highest standards in modern manufacturing. Manufacturers with high-quality production environments easily distinguish their products from low-cost substitutes in the marketplace. For e-liquids, USP-grade products represent pharmaceutical precision throughout every stage of production and packaging, including analysis for purity and potency, lot-to-lot tracking, and in the U.S. regulated by FDA oversight.
Reduce Risk for Immunocompromised Users
In patients undergoing or recovering from cancer therapies, autoimmune diseases, organ transplants, or renal therapies, exposure to any amount of opportunistic pathogens and fungi represent grave risks for complications, slower recoveries, elusive diagnosis, less effective therapies, reduced survival rates, death, and unforeseen complications.
A 2015 study indicates that microbial contamination is not widespread among testing of readily available e-liquids. These studies are relatively limited in scope and sample size. Meanwhile the FDA will shade towards caution when considering the vast audience and market growth expected for e-liquid products, Forecasts expect the market to nearly double its value from $11.5B in 2018 to $24B by 2024.
Propylene glycol (PG) mixtures are sometimes represented as “antimicrobial” suggesting that e-vape solutions are unable to host or transfer bacteria. Contrarily, studies show that PG is only marginally bacteriocidal. While the solution may reduce colony growth between 25% – 50%, this does not constitute antimicrobial efficacy, nor does it afford safe outcomes for immunocompromised patients. The future of FDA regulated e-liquid manufacturing will, without question, require stringent measures against opportunistic bacteria and viruses.
Standardization of E-Liquid Manufacturing
The International Standards Organization (ISO), United States Pharmacopeia (USP) and The American E-Liquid Manufacturing Standards Association (AEMSA) are examples of non-government standards which create transparency for manufacturing expectations and quality control. These standards signal quality, consistency, and product safety thus fetching higher premiums at market.
The American E-Liquid Manufacturing Standards Association
The American E-Liquid Manufacturing Standards [AEMSA] is one of the most widely accepted benchmarks for E-liquid standards. It iterates many of the values synonymous with other safe manufacturing methodologies as follows:
- Verify the accuracy of any nicotine content in the products we distribute.
- Ensure the quality of all ingredients in our e-liquids.
- Prepare our products in a clean, sanitary and safe environment.
- Ensure our products are packaged and delivered in a safe manner.
- Provide a level of transparency in the monitoring and verification process.
ISO Cleanroom Manufacturing Standards for E-Liquids
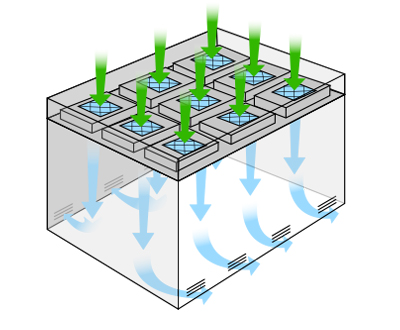
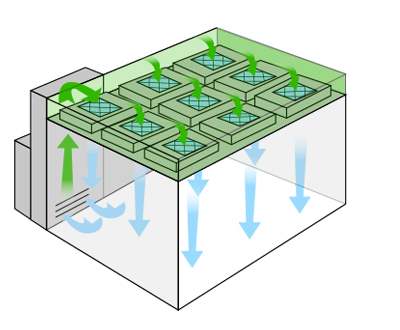
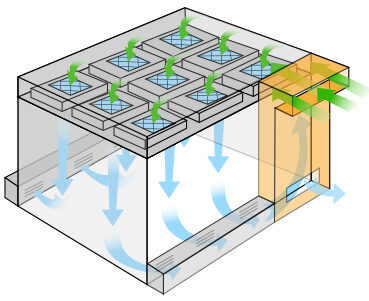
Most e-liquid and e-cigarette cleanrooms range between an ISO 6 – an ISO 8. The air change rate is one way of benchmarking the final performance and cleanliness of the room as installed. Hygiene, equipment, number of workers, and production volume all influence the real-time operating conditions. Air is either recirculated and reintroduced in recirculating designs; or introduced and vented outward within “pass-through” designs. Directional airflow, low-lint materials, and high-performance HEPA air filtration systems prevent build-up or retention of microbes, allergens, mold spores, and particulates.
USP – United States Pharmacopoeia
USP certification is a common standard among premium e-liquid ingredients. USP products face stringent manufacturing controls, analysis for ingredient purity, lot-to-lot tracking, and FDA oversight. This standard is rather rigorous, which details specific ISO classification requirements based on the risk and sensitivity of the product. We’ve seen some recommend USP standards for E-liquid productions and labs, however, it’s best to take these recommendations with a grain of salt. You’ll only require a USP cleanroom if you intend to produce USP-grade products.
Good Manufacturing Practices for E-Liquids
What is GMP?
Good Manufacturing Practices normalize production cleanliness, labeling standards, and best practices for licensed and authorized products. Associated industries include food and beverage, cosmetics, pharmaceuticals, dietary supplements, medical devices, and recently tobacco-related delivery systems. These recommended procedures and standards ensure that products maintain true-label ingredients and characteristics, such as identity, strength, composition, ingredient quality, and purity.
How Clean is A Cleanroom?
Before we consider the cleanliness of a cleanroom, we must first compare a “non-cleanroom”, similar to the one you’re sitting in right now.
Airborne particle counters allow extremely precise measurement of micro particulates otherwise undetectable by the naked eye. Test results from our office conference room indicated roughly 22 million particles per. cubic meter as the HVAC clicks on, and about 16 million particles per cubic meter once the HVAC clicks off the room settles.
For reference, an office environment with a commercial HVAC system recirculates the entire volume of air in the building somewhere between 2 – 6 times an hour. In an ISO 8 cleanroom, room air is completely circulated somewhere between 10 – 20 times per hour.
So how much cleaner is an ISO 8 cleanroom (the least-clean cleanroom) than a standard office environment?
Based on airborne particle counts, the ISO 8 room is cleaner by three orders of magnitude — even when compared to a relatively small, low-traffic conference room — an ISO 8 production area contains 10 times fewer particulates of 0.5 µm or larger than the cleanest non-controlled area. For each drop in ISO Class, cleanliness improves by another factor of 10.
Read More: Understanding Cleanroom Air Change Rates
What We Like About Smart Features from Particles Plus
Local communication protocols such as Bluetooth, Zigbee, JSON, and other communication links provide onboard capabilities for syncing environmental monitoring and testing tools with Cloud connections. These particulate and air quality sensors are now available in handheld and remote units which are controlled from web browsers or on-board display. Learn more about how these particle counters allow real-time adjustment, remote monitoring, and data visualization.
Read More: What Is Particulate and How Do You Test It?
ISO Classes for E-Liquids
The difference in air quality between an ISO 8 and ISO 7 cleanroom is significant, roughly a factor of ten. Most E-liquid manufacturers opt for an ISO 7 or ISO 8 cleanroom for direct manufacturing and compounding of solvents, solutes, and raw ingredients. Post-processing, packaging, labeling, or inspection are feasible in less constricted areas with less intensive air requirements, usually ISO 8.
| ISO Class Maximum Particles m3 | ≥0.5µm | ≥1µm | ≥5µm |
| ISO 5 (Critical Electronics) | 10,200 | 3,520 | 832 |
| ISO 6 (Electonronics) | 35,200 | 8,320 | 293 |
| ISO 7 (Liquid Processing) | 352,000 | 83,200 | 2,930 |
| ISO 8 (Packaging) | 3,520,000 | 832,000 | 29,300 |
| ISO 9 (Room Air) | 35,200,000 | 8,320,000 | 293,000 |
An ISO 7 cleanroom is a pharmaceutical-grade environment suitable for processing, packaging, and compounding medical-grade products in a positive pressure environment. While these environments are not sterile, they support aseptic workflow, a specific workflow that minimizes the retention of particulates and opportunistic microbes.
Most Common Contaminants in E-Liquids
The most problematic contaminants include chemically nefarious ingredients, such as carcinogens, residues, and solvents. Diethylene glycol, ethylene glycol, hydrocarbons, aldehydes, acetone, and ethanol present sliding scales of risk and potential toxicity. Risk factors compound when mixed ingredients interact during combustion or when exposed to light and high temperatures during storage. Airflow is directional, which minimizes temperature gradients, predicates static events, stabilizes vapor expansion, and perseveres gas-liquid states.
Cleanroom Design for E-Liquid Labs & Facilities
Cleanroom design and construction is both science and art. The scenarios and information herein provide a road map considering a cleanroom production environment for e-liquids, components, packaging, accessories, and other ENDS (electronic nicotine delivery systems).
End-user risk, operating costs, and expected return on quality control investments provide starting points for e-liquid cleanrooms. Consulting with a cleanroom specialist is the fastest way to narrow down the things your facility needs, but also the things you don’t.
For example, will you need a gowning room? Does the structure need to fit through standard doors? Is the installation something your comfortable handling on your own?
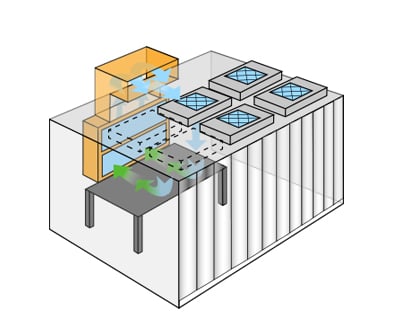
Gowning Rooms and Clean Areas
Every cleanroom workflow starts outside of a cleanroom. A ‘gowning room” — (also called and ante-room) — provides a changing area for donning cleanroom rated clothes and PPE. Hygiene regimens are often well documented with established procedures for hand washing and posted standards for well-kept nails, garbing order, and tying/covering hair. For the most sensitive cleanrooms, even sinks, mirrors, dispensers, and lockers require cleanroom rated materials. Sequentially located dispensers, intuitive floor markings, and clean/dirty areas foster consistency and product fewer mixups.
Cleanroom Packaged Materials
Consumable items are generally cleanroom packaged and ready to use, or support sterilization/disinfection before passage into the cleanroom. All of our cleanroom products are tested and validated for cleanroom use. That doesn’t mean that every product is appropriate (or cost-effective) for every cleanroom or task. Sometimes, static sensitivity, chemical resistance, sterility, or low particulates are more or less of a priority.
E-Liquid Clean Room Wall Systems Design
Hardwall E-Liquid Cleanrooms

We offer multiple cleanroom construction types, panel styles, surface materials, and more in virtually unlimited configurations and floor sizes. Each style of cleanroom panel, HVAC system, and structural design is unique in terms of its surface options, final height, assembly method, and its structural capacity.
Read More: Advantages of a Hardwall Cleanroom System
Hardwall cleanrooms are rigid wall containments with either A) heavy-duty structural walls or B) more lightweight “insert style” walls. Structural hard walls support load bearing of roof-mounted generators, HVAC systems, or mezzanines. Insert style panels offer lightweight structures that require only a forklift for setting ceiling beams.
Softwall Cleanrooms Systems
Softwall cleanrooms, enclosures, or doorway fixtures substitute solid panels for “plastic curtains” or “plastic strip doors”. Strip curtains and fan filter units affix with hardware after the frame is erected. It’s an economical option that requires little to no pre-engineering and supports most non-critical clean operations such as handling and packaging applications.
Softwall cleanroom systems either isolate a clean process or contain high-particulate or vapor prone processes such as paint, powder, or packaging. The structural support system is lightweight and available with wheels/casters. Many facilities opt for self-installation, as the frames and curtains assemble with simple hardware and do not require welding.
Suspended isolation systems allow direct attachment of a softwall system to an existing ceiling grid. This can be a cost-saving and flexible option for containment structures.
The most obvious advantage of softwall cleanroom is affordability. Relatively small curtain containments need not exceed a few thousand dollars. The cost includes not only the structure but also the fan filtration units, ceiling, curtains. Installation requires only hand tools, no welding.
The downside of a softwall system is that its far less impervious to inbound contamination. Because the room does not have sealed sidewalls, it exhausts air outwardly through the curtains and does not recirculate. Operating temperature and humidity is less consistent. The room requires increased airflow for positive pressure states. A softwall cleanroom may achieve classification as low as ISO 6 via sealed, HEPA outfitted fan blower units. Most facilities with softwall systems do not require a specific ISO classification.
Softwall E-Liquid Cleanroom Principles
Suspended Curtain Isolation Systems
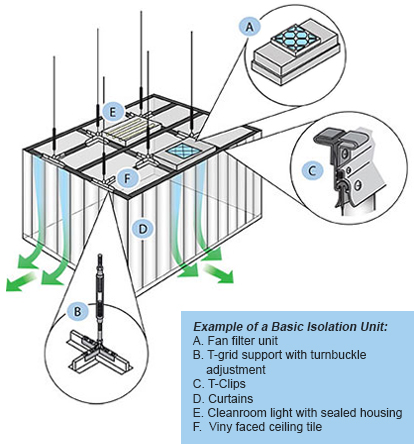
- Powered fan filter unit draws the air from above the clean space.
HEPA filtered air pressurizes the clean space keeping particles out. - Downward velocity pushes any particulate generated in the room to the floor and out of the space.
- 2″ heavy-duty t-grid supported from the existing ceiling every 4 feet.
- Easy leveling with rod and turnbuckle adjustment.
- T-clips allow a curtain to be hung from the t-grid. This can make a wall or partition within the clean space.
- Curtains with an RF welded hook bead make installation easy.
- The air gap at the base of the curtain is engineered to allow the right level of exhaust air.
Standalone Softwall Cleanroom
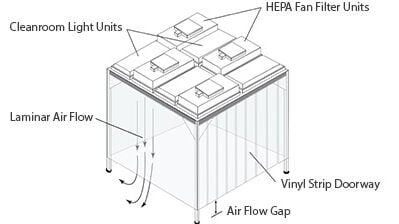
Steel Frame units are made of tubular steel components. The components are cut, formed, welded and powder coated. The frames are bolted together to create the room.
Aluminum Frame units are made out of Extruded Aluminum profiles with an anodized finish. They are assembled using internal clinch lock fasteners, no additional hardware is required.
Pros
- Lowest overall cleanroom cost per square foot (if HVAC required)
- Standard size units can ship in as little as two weeks.
- Complete units can be bolted together to make larger clean areas.
Containment Zone
A pressured containment zone with HEPA filtered air is common when a use case does not require a specific ISO classification, but still requires containment or processing area.
Garments & PPE Requirements for E-Liquid Cleanroom Manufacturers
Gowning and PPE for E-Liquid Cleanrooms
Gowning and PPE requirements when packaging or handling tobacco products vary based on the substance at hand. For e-liquid tasks requiring pure, non-diluted nicotine, a head to toe suit includes chemical barrier gloves, gowns, hoods, gloves, shoe protection, and masks/goggles.
In less intensive cleanrooms for packaging or labeling, the minimum attire is usually a hairnet, a lab coat/frock, and shoe covers.
Section 3.09 Employee Safety (AEMSA)
The American E-Liquids Manufacturing Standards provides these guidelines for e-liquid PPE:
(a) Employers MUST provide their employees with a workplace that does not have serious hazards and follow all relevant local, state, and federal requirements including – but not limited to – the following mandatory personal protective equipment (P.P.E.):
(i) Eye protection must meet or exceed ANSI Z87.1 standards and must be worn at all times in process areas of the manufacturing environment.
(ii) Lab Coat / Apron
(i) Long sleeves required. If an apron is chosen for PPE, appropriate long-sleeved undergarments must be chosen so that the entire arm is covered from environmental hazards during the manufacturing process.
(iii) Full coverage pant/leg attire
(i) Garment(s) must not be torn or contain opening(s) that may expose workers to environmental hazards during the manufacturing process.
(iv) Fully covered footwear
(v) Chemical-resistant / liquid-resistant gloves
(vi) All manufacturing spaces must have easily accessible
- First aid kit
- Emergency eyewash kit
- Chemical containment spill kit
Compare top brands and get bulk discounts. Need helping determining the proper garment or equipment for your gowning room? We’re your controlled environment specialist with dedicated supply chain support.
Other Contamination Controls
Decontamination mats (sticky mats) and air showers (air interlocks) provide permanent barriers against clothing borne bacteria, spores, pollen, and sometimes, even insects and pests. Likewise, any equipment with wheels such as carts or pallet jacks can also pass through extra-wide air showers.
Chemicals & Cleaning
Cleanroom approved wipes, chemicals, and garments reduce lint shedding during wipe down and sanitation. Automated dispensers and streamlined floorplans reduce traffic jams, prevent confusion, and encourage proper garbing order before and after completing work.
Static Control Areas
Static control is important when handling or manufacturing any sensitive electronic devices, such as e-cigarette vaporizer components or assemblies. One must consider that most manufacturing operations favor dry environments with relatively low relative humidity RH. Dry air is beneficial for most packaging operations, as well as drying or curing between or after production stages. Trace moisture is problematic for nearly any assembly or production process, however dry air is problematic for static generation.
Dry conditions increase the likeliness for static generated charges. Static discharge is not only a risk to electro-static sensitive devices (ESDS) devices, but also attracts micro-sized dust and particle clumps. For more information on the static management systems and equipment, view our static control knowledge base.
Read More: ESD and Static Control Cleanrooms
What is the Best E-Liquid Cleanroom for You?
The fundamental goal of each cleanroom is unique to its process, and so is the science behind its design. For most facilities, speaking with a controlled environment specialist is the fastest path from clean idea to a clean build.
PAC cleanroom specialists help you identify which components best suit for your application with respect to federal and organizational guidelines. Let’s connect you to one of our modular cleanroom solutions, or provide you with a free quote for a custom cleanroom.

Cleanroom Solutions
Keep Reading
Related Posts
-
Everything You Need to Know About Building A Cleanroom: ISO Class Prefab Hardwall Cleanroom Design
Advantages for Modular Hardwall Design and Construction of ISO 14644-4 Cleanrooms.
-
FDA Regulation of E-Liquid Manufacturing
While most large scale production facilities have standardized manufacturing controls, those mixing liquids in small facilities without environmental controls may struggle to receive pre-market approval.
-
Cleanroom Construction FAQ
Definitive Cleanroom Construction FAQ: How much does a cleanroom cost? How does the level of ISO classification impact build and operating costs? How much supporting space will I need for a cleanroom addition or ISO…
-
CleanPro® Softwall Cleanroom Enclosure
This customer needed to enclose a piece of machinery, and CleanPro® was able to provide a solution.
-
CleanPro® Turnkey Cleanroom Install
CleanPro’s turn-key cleanroom solution provided a one-stop, one-contact result for the initial delivery and on-site installation of walls, ceiling grids, electrical systems, flooring, filters, HVAC, and more.
-
CleanPro® Chemotherapy & CSP Cleanroom Installation
This modular, sterile compounding cleanroom is designed for USP 797 and USP 800 compliance, particularly for compounding chemotherapy drugs. Safe handling of sterile compounds requires special considerations: heat-welded floors, anterooms and buffer areas.
-
CleanPro® Softwall Cleanroom Curtains Installation
Softwall cleanroom curtains, sometimes referred simply as “plastic strips” yield ISO Class 10,000 level particle control with minimal construction. This customer required a custom softwall installation that integrated into their existing building structure CleanPro to…
-
Cleanroom Components — Air Filtration, Design, and Other Variables
What are the key determinants of cleanroom components? Learn about different types of cleanroom filtration, wall construction, lights, temperature, humidity and more.
-
FS209E and ISO Cleanroom Standards
Before global cleanroom classifications and standards were adopted by the International Standards Organization (ISO), the U.S. General Service Administration's standards (known as FS209E) were applied virtually worldwide. However, as the need for international standards grew,…
-
How to Choose the Right Cleanroom Particle Counter
Cleanroom particle counters differ widely based upon particle threshold, cost, and features. How to do you determine if you need a particle counter or what model of particle counter to purchase? In this article we…
-
Modular Cleanrooms for Business Startups & New Product Development
Regulations, Cost, Location, Size, Performance – How Do You Choose the Right Cleanroom? Selecting a cleanroom for a new business or product is not a difficult process. There are many considerations and options, but focusing…